

Affiliations
ABSTRACT
Renal Malignant Epithelioid Angiomyolipoma (RMEAML) is a rare subtype of angiomyolipoma (AML) with malignant potential. We present two case reports of a 20-year boy and a 46-year female who were diagnosed with RMEAML after presenting with flank pain and haematuria at our institution in the same year. The case reports are presented to analyse their clinical behaviour and histopathological features, and discuss diagnostic pitfalls. Both patients underwent radical nephrectomy, and the histopathology report revealed the presence of RMEAML with high-grade features. Later, these patients were referred for adjuvant therapy, and currently, they are under regular follow-up. The case reports highlight the importance of considering RMEAML in the differential diagnosis of renal tumours with atypical features. Diagnostic imaging alone cannot reliably differentiate between benign AML and RMEAML, and a histopathological examination is mandatory to confirm the diagnosis. Early diagnosis and prompt treatment are crucial in the management of RMEAML, considering its malignant potential.
Key Words: Adjuvant therapy, Radical nephrectomy, Renal Malignant Epithelioid Angiomyolipoma, Histopathology.
INTRODUCTION
A distinctive subtype of Perivascular Epithelioid Cell Tumours (PEComas) is Epithelioid Angiomyolipoma (EAML). EAML, an infrequent type of renal Angiomyolipoma (AML) with malignant potential, is characterised by a predominance of HMB-45 positive epithelioid cells, accompanied by the notable absence of adipocytes. Although its presence had previously been questioned, malignant EAML has lately been described as an uncommon kidney tumour.1 The literature contained several reports of EAML cases that metastasised to the liver, lung, and bone.2,3 The histological findings that supported the diagnosis were also supported by the presence of many metastases. Due to the resemblance of EAML's epithelioid morphology to Renal Cell Carcinoma (RCC), the diagnosis may be difficult.4 However, it may be easier to identify this entity if one is aware of its traits, such as its immunoreactivity to HMB-45. The pathological findings and clinical behaviour of malignant renal EAML may be better understood with the aid of the classification criteria for these malignancies that are presented in the current study. In both instances, the patients’ families gave their written, informed consent.
CASE REPORTS
CASE 1:
A 20-year boy, a student, presented with a history of flank pain on the left side for about 5 to 6 months. One month ago, he developed haematuria. An ultrasound of kidney, ureters, and bladder (KUB) was performed, revealing a heterogenous rounded soft tissue lesion at the mid-pole of the left kidney with internal cystic areas measuring 8 × 9.2 cm. A Computerised Tomography (CT) scan of the abdomen was also conducted, showing a mass lesion in the left renal upper pole, likely renal cell carcinoma (T3aN0Mx). It was enhancing on contrast enhancement with central necrosis and measured 7.9 × 8.8 cm (AP × T), causing a mild mass effect on the spleen. The internal necrotic part contained areas of hyperdensity on the non-contrast images, suggesting haemorrhage. The mass extended into the renal sinus fat, with marked indentation on the interpolar region calyces, and the possibility of involvement of these calyces by this lesion could not be excluded (Figure 1). The left renal vessels and left adrenal were unremarkable. The lung bases, liver, gallbladder, right kidney, right adrenal, pancreas, bowel, pelvic organs, soft tissues, and bones were unremarkable. No significantly enlarged lymph nodes or ascites were observed. A CT chest was recommended for the completion of staging, which showed no focal pulmonary lesions in the lungs, mediastinum, axillary, and supraclavicular regions. The DTPA scan showed left kidney function of 45% with mild resistance to urinary flow and right kidney, 55% split function, whereas the laboratory investigations showed a serum creatinine of 1.0 mg/dL. The patient successfully underwent left laparoscopic radical nephrectomy and had an eventful recovery. The histopathology showed the presence of >80% of epithelioid cell morphology. The neoplasm was composed of cells with voluminous eosinophilic to clear cytoplasm arranged in a predominantly alveolar pattern. The cells showed marked nuclear atypia with abundant mitoses. Some areas of tumour necrosis were also seen. The section from the renal vein showed a tumour thrombus. The immunohistochemistry (IHC) showed a positive expression of HMB-45 and Cathepsin K. However, the tumour was negative for various other markers, including Desmin, Smooth Muscle Actin (SMA), PAX8, Calretinin, Inhibin, Vimentin, S-100, Carbonic Anhydrase IX (CA-IX), Cytokeratin 7 (CK7), CD117, Epithelial Membrane Antigen (EMA), Synaptophysin, Chromogranin, and CK8/18. Additionally, special stains revealed positive Periodic Acid-Schiff (PAS) staining in vessels and the basement membranes, while iron staining was negative (Figure 2: a-h). After four months of surgery, the patient came again with an acute complaint of left flank pain. The case was discussed in a multi-disciplinary team (MDT) meeting, as there was a suspicion of recurrence, and it was decided to follow up with the patient by doing 6-monthly CT of chest abdomen and pelvis.
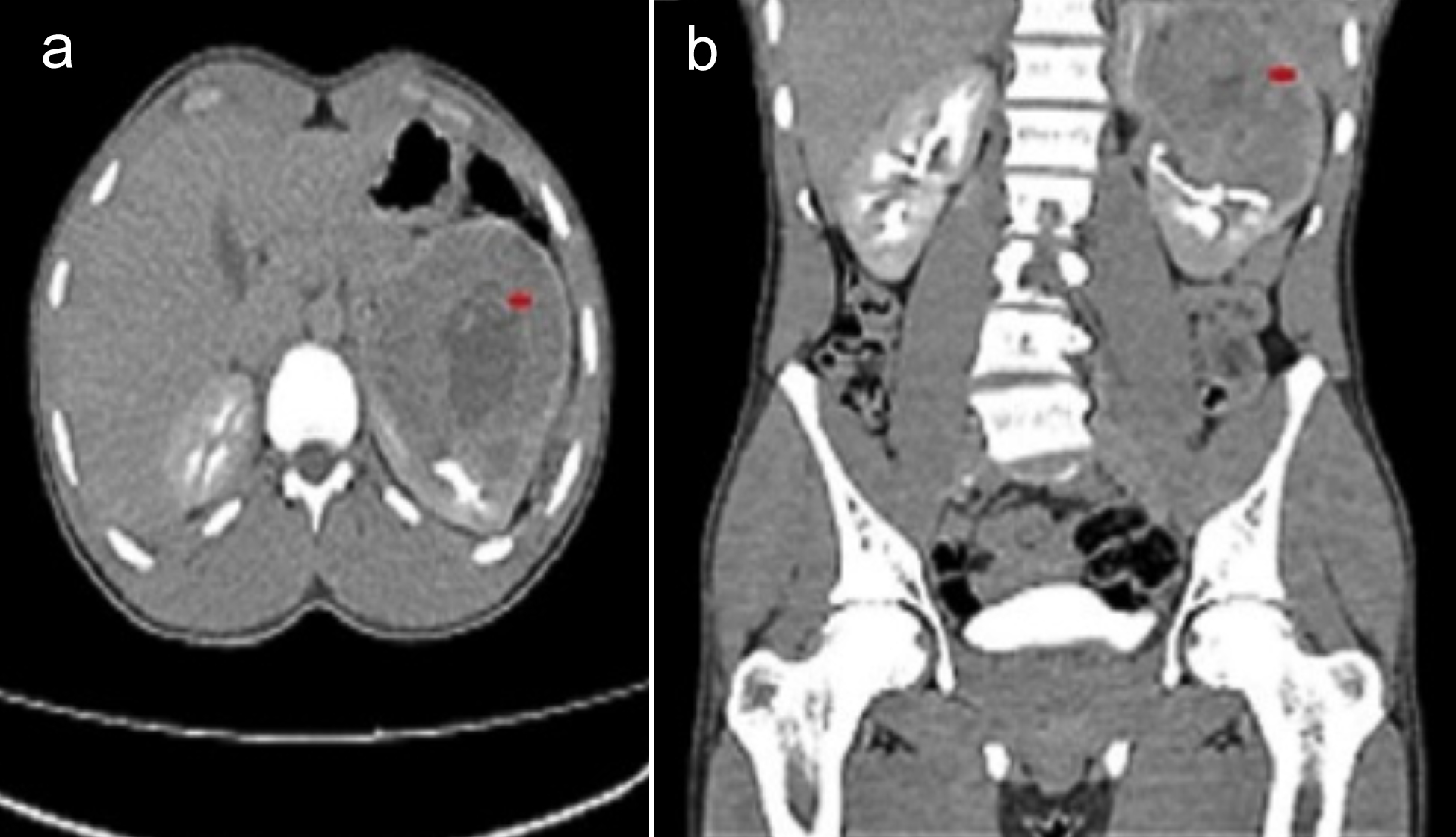 Figure 1: CT abdomen; (a) Transverse view: The red arrow shows a left renal upper pole mass lesion, likely renal cell carcinoma; (b) Coronal view: The red arrow shows a left renal upper pole mass extending into the renal sinus fat, with marked indentation upon the interpolar region calyces.
Figure 1: CT abdomen; (a) Transverse view: The red arrow shows a left renal upper pole mass lesion, likely renal cell carcinoma; (b) Coronal view: The red arrow shows a left renal upper pole mass extending into the renal sinus fat, with marked indentation upon the interpolar region calyces.
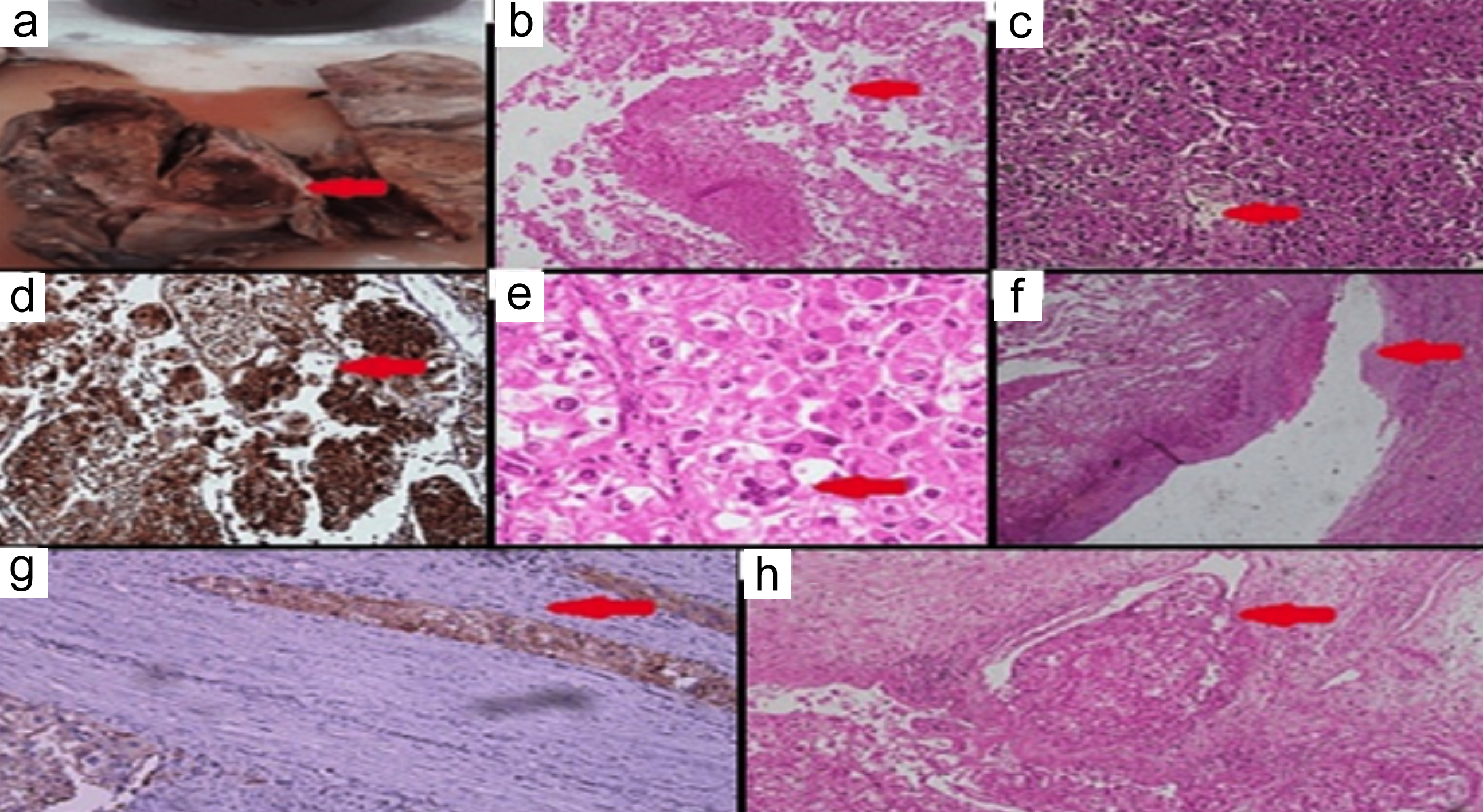 Figure 2: Histopathological and immunohistochemistry images; (a) Red arrow shows gross section, (b) Red arrow shows area of necrosis ×10, (c) Red arrow shows carcinoma-like growth pattern, ×20, (d) Red arrow shows HMB-45 positivity in tumour, ×10, (e) Red arrow shows nuclear pleomorphism with multinucleation, ×40, (f) Red arrow shows tumour thrombus in renal vein, ×20, (g) Red arrow shows Cathepsin K focal positivity in tumour, ×20x, (h) Red arrow shows tumour involving renal sinus fat, ×10.
Figure 2: Histopathological and immunohistochemistry images; (a) Red arrow shows gross section, (b) Red arrow shows area of necrosis ×10, (c) Red arrow shows carcinoma-like growth pattern, ×20, (d) Red arrow shows HMB-45 positivity in tumour, ×10, (e) Red arrow shows nuclear pleomorphism with multinucleation, ×40, (f) Red arrow shows tumour thrombus in renal vein, ×20, (g) Red arrow shows Cathepsin K focal positivity in tumour, ×20x, (h) Red arrow shows tumour involving renal sinus fat, ×10.
On the follow-up, a single venous phase contrast-enhanced CT scan of the chest, abdomen, and pelvis was done as decided in the MDT meeting. The CT revealed a large soft tissue density mass in the left renal surgical bed with adjacent extensive left retroperitoneal and intraperitoneal soft tissue deposits, also involving the subscapular surface of the liver and porta hepatis. These findings were concerning regarding disease recurrence at the surgical bed with retroperitoneal, peritoneal, and para-aortic nodal metastases. Lungs were clear of collapse, consolidation, or size-significant pulmonary nodules (Figure 3). Due to the suspicion of recurrence, it was decided to refer the patient to an oncologist for further management. He is currently under regular follow-up.
CASE 2:
A 46-year married female with two children with no comorbidities presented for the management of an exophytic neoplastic mass at the right renal upper pole, measuring 9 cm, with regional extension on contrast-enhanced CT chest abdomen. The patient reported a 2-3 month history of fever and vomiting immediately after eating or drinking. She experienced pain localised on the right side, extending into the epigastrium, and had a weight loss of from 56 kg to 47 kg over a few months, along with haematuria.
The laboratory investigations revealed a haemoglobin of 5.6 g/dl (Reference range: 12-15 g/dl), alkaline phosphatase (ALP), 351 U/L (Reference range: 37-98 U/L), γ-Glutamyl Transferase (GGT), 151 g/dl (Reference range: 12-64 g/dl), Aspartate Transferase (AST), 52 U/L (Reference range: 5-34 U/L), Alanine Transaminase (ALT, 59 U/L (Reference range: 0-55 U/L), and globulin at 3.8 g/dl (Reference range: 2-3.5 g/dl).
A surgical intervention was planned. Preoperative shortness of breath was noted, for which HRCT chest as well as chest x-ray were performed on the advice of a pulmonologist. The imaging showed diffuse ground glass haze with mild interlobular septal thickening, mild bilateral pleural effusions, and mild pericardial effusion. Cardiac size was enlarged, and hilar vascular congestion was noted. The patient underwent right laparoscopic radical nephrectomy. A macroscopic examination of the specimen showed a tumour involving the upper pole of the kidney, measuring 9 × 8 × 6 cm, with a haemorrhagic and necrotic cut surface, having small cystic areas, and grossly abutting and involving the renal sinus fat. The microscopic examination revealed a malignant neoplasm with >80% epithelioid morphology, involving renal parenchyma composed of sheets and nests of large polygonal cells with abundant eosinophilic cytoplasm, marked nuclear pleomorphism, and large prominent nucleoli.
 Figure 3: Single venous phase contrast-enhanced CT scan of chest, abdomen, and pelvis; (a) Red arrow shows disease recurrence at surgical bed with retroperitoneal, peritoneal, and para-aortic nodal metastasis, from previously resected primary malignant left renal tumour; (b) Red arrow shows large soft tissue density mass in left renal surgical bed with adjacent extensive left retroperitoneal and intraperitoneal soft tissue deposits.
Figure 3: Single venous phase contrast-enhanced CT scan of chest, abdomen, and pelvis; (a) Red arrow shows disease recurrence at surgical bed with retroperitoneal, peritoneal, and para-aortic nodal metastasis, from previously resected primary malignant left renal tumour; (b) Red arrow shows large soft tissue density mass in left renal surgical bed with adjacent extensive left retroperitoneal and intraperitoneal soft tissue deposits.
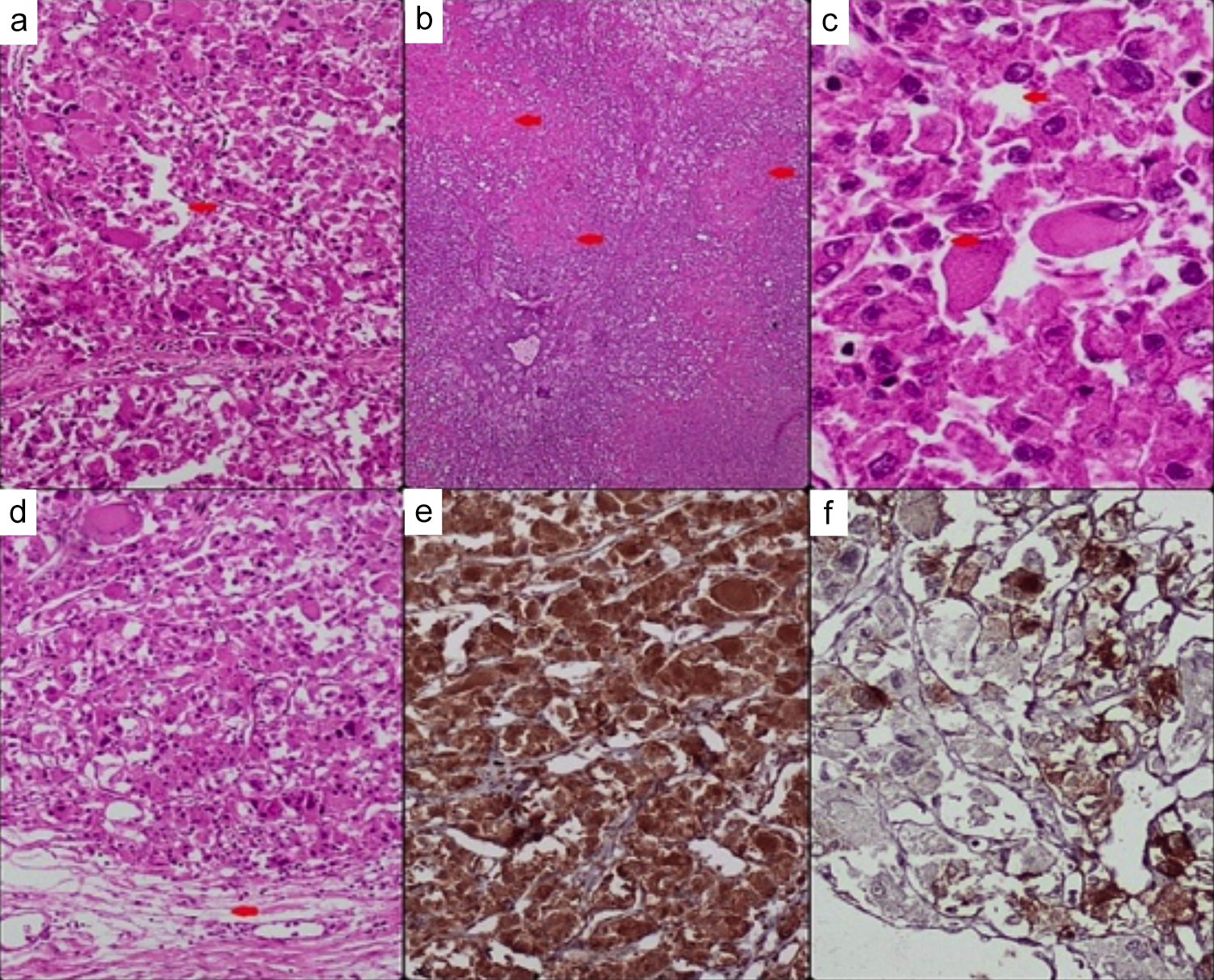 Figure 4: Histopathology and immunohistochemistry images; (a) Red arrow shows carcinoma-like growth pattern, ×10, (b) Red arrows shows geographic areas of necrosis, ×4x, (c) Red arrows shows neoplastic cells with abundant eosinophilic cytoplasm, multinucleation, intranuclear inclusions and prominent nucleoli, ×40x, (d) Red arrow shows renal sinus hilar invasion, ×10, (e) Cathepsin K positive ×10, (f) HMB-45 positive in some tumour cells, ×20.
Figure 4: Histopathology and immunohistochemistry images; (a) Red arrow shows carcinoma-like growth pattern, ×10, (b) Red arrows shows geographic areas of necrosis, ×4x, (c) Red arrows shows neoplastic cells with abundant eosinophilic cytoplasm, multinucleation, intranuclear inclusions and prominent nucleoli, ×40x, (d) Red arrow shows renal sinus hilar invasion, ×10, (e) Cathepsin K positive ×10, (f) HMB-45 positive in some tumour cells, ×20.
Many cells showed multinucleation, and numerous atypical mitoses were present. Some extensive areas of necrosis were also seen. The tumour was infiltrating the renal sinus fat with no lymphovascular invasion. The attached benign right adrenal gland was also seen compressed by the tumour; however, the tumour did not infiltrate or invade the adrenal gland. IHC results revealed diffuse positivity for Cathepsin K, with focal positive staining for HMB-45 and Melan-A. Negative staining was observed for PAX8, RCC antigen, CK7, CD10, CD117, Carbonic Anhydrase IX (CAIX), Desmin, Myogenin, SMA, Calretinin, Inhibin, and S-100. The special stain, PAS, was also negative (Figure 4: a-f). A new chest x-ray was performed, and a comparison was made with the previous radiograph, in which the right approach central line was noted in situ lungs were clear of any focal lesion or consolidation. The left costophrenic (CP) angle was not visualised clearly. The cardiac size was enlarged. The trachea was central in position. The visible skeleton and overlying soft tissues appeared unremarkable.
An ultrasound was performed to assess the hepatobiliary system due to elevated liver function tests (LFTs). The liver revealed normal parenchymal echotexture with smooth margins, and no focal liver lesions were observed. The Common Bile Duct (CBD) was slightly prominent for the patient's age, measuring 5.5 mm at porta hepatis; however, no intrahepatic biliary dilatation was noted. The gallbladder was distended. After two and a half months post-surgery, a CT renal angiogram, including arterial, venous, and delayed phases, was conducted. The results indicated a well-circumscribed fat density area in the right suprarenal region, likely representing a residual lesion, though no solid-enhancing components were identified. The small left lower lobe pulmonary nodule remained unchanged, requiring follow-up and comparison with previous studies. The patient was referred for chemotherapy to a specialised institute, along with a plan for 3-monthly CT scans and renal function tests (RFTs).
DISCUSSION
In contrast to EAMLs, which are exceptionally rare and constitute only 4.6% of consecutively resected AMLs, classic AMLs account for 1% of surgically removed renal tumours.5 EAML exhibits an equal sex predilection and a mean age of 40 years. There are no distinguishing characteristics between EAML and traditional AML in terms of clinical symptoms or imaging modalities. These tumours are typically solid and haemorrhagic, as observed in both of our cases. Two architectural patterns, namely AML with a carcinoma-like growth pattern, characterised by large cells arranged in cohesive nests, and AML with epithelioid spindled cells organised in a diffuse growth pattern, can be identified in EAML under the microscope.6
The epithelioid component in these cases represents a histological subtype characterized by cells exhibiting epithelial characteristics, such as prominent cell borders, distinct cell arrangements, and recognizable cytoplasmic features. Histologically, RCC or melanoma may be confused with EAML, especially when an epithelioid component and nuclear atypia are visible. Strong melanocytic markers (HMB-45, Melan-A) and SMA immunostaining are observed in AML. Radical tumour excision may be crucial for the course of treatment. Although the effects of adjuvant radiochemotherapy should be further studied, they may be advantageous.7 About 17% of patients experienced recurrence, and 30% of patients experienced metastasis, as was the situation in our first case.8 The lymph nodes, liver, lung, and peritoneum were the most typical metastatic sites. In individuals who develop metastatic disease during follow-up, the median period to metastases is 17-31 months.9 There is a lack of clarity on the prognostic variables indicating malignancy in EAML. Associated Tuberous Sclerosis Complex (TSC), tumour size (>7.7 cm), extra-renal extension or renal vein invasion, carcinoma-like growth pattern, tumour necrosis, percentage of abnormal epithelioid cells (70%), >2 mitotic figures/10 HPFs, and atypical mitoses are some of these.10 However, the diagnosis of malignancy should only be based on the occurrence of metastases.
Although each of the two cases fulfilled six of the criteria, one of them displayed signs of malignant clinical behaviour at the time of the available follow-up.
The imaging findings of the EAML can overlap with those of other, more common renal neoplasms. Therefore, any significant renal mass with the following histological characteristics should be evaluated as part of the differential diagnosis: minimal fat, necrosis, and bleeding. There are significant morphological similarities between EAMLs and more typical kidney neoplasms. An appropriate immunostaining with melanocytic markers is necessary for an accurate diagnosis.
EAML can exhibit aggressive behaviour and potentially metastasize. The variety of poor prognostic criteria observed appear to relate to the course of the disease. An adjuvant therapy might be required if the surgical resection alone is insufficient. To establish a standardised therapy for this form of AML, a multimodality therapeutic strategy needs to be investigated.
PATIENTS’ CONSENT:
Informed written consents were taken from the patient or the patient’s family.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
NZ, NBN, AR, SM: Study concept and design, data collection.
SI: Drafting and critical revision of the manuscript.
All authors approved the final version of the manuscript to be published.
REFERENCES